Answer:
The daily production rate is 191.6 ton/day
Step-by-step explanation:
The first step that is required to be carried out is by determining the thermal input:
The thermal input can be calculated via the expression:
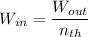
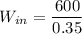
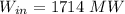
The feed rate is calculated as:
coal feed rate =
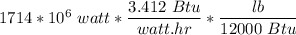
= 487347 lb/hr
The sulfur feed rate is :
= 487347 × 0.035
= 17057 lb/hr
Sulfur removal rate = 17057 × 0.9
= 15351 lb/hr
However, to determine the actual alkalinity; we have:
actual alkalinity = 1.15 × 239.57
= 275.5 lb . mol / hr
The total alkalinity is =
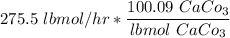
=
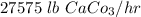
The limestone feed rate =
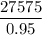
= 29026 lb/hr
=
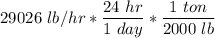
= 348.3 ton/day
Finally, the daily production rate = 0.55*348.3 ton/day
= 191.6 ton/day