Answer:
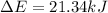
Step-by-step explanation:
Hello,
In this case, we should apply the first law of thermodynamics to compute the energy change:
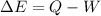
Thus, with the given volume change we compute the corresponding work in kJ:
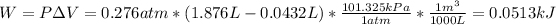
Then, we compute the energy change:
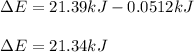
Best regards.