Answer:
The new volume of the gas is 21 L.
Step-by-step explanation:
Volume of a gas is inversely proportional to its pressure at constant temperature such that,
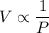
or

We have,
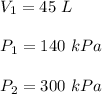
It is required to find V₂. Using above law or Boyle's law such that :
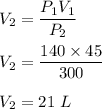
So, the new volume of the gas is 21 L.