Answer:
The final temperature of the water is 41.05 degrees celsius.
Step-by-step explanation:
We have,
Mass of sample of water is 221.7 g
Initial temperature was 25.0 degrees Celsius
Heat produced in it is 3560.6 calories of heat
The specific heat capacity of water is 4.186 J/g °C
1 calorie = 4.184 J
3560.6 calorie = 14897.55 J
The heat produced due to change in temperature is given by :
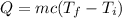
c is specific heat capacity
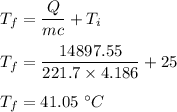
So, the final temperature of the water is 41.05 degrees celsius.