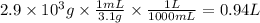Answer:
0.94 L
Step-by-step explanation:
Step 1: Write the balanced reaction
2 Al + 3 Br₂ = 2 AlBr₃
Step 2: Calculate the moles of Br₂ required to produce 12 moles of AlBr₃
The molar ratio of Br₂ to AlBr₃ is 3:2. Then,
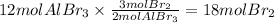
Step 3: Calculate the mass of bromine corresponding to 18 moles
The molar mass of bromine is 159.81 g/mol. Then,
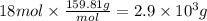
Step 4: Calculate the volume of bromine corresponding to 2.9 × 10³ g
The density of bromine is 3.1 g/mL. The volume of bromine is: