Answer:

Step-by-step explanation:
The balanced chemical reaction is,
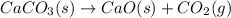
The expression for Gibbs free energy change is,
![\Delta G_(rxn)=\sum [n* \Delta G_(product)]-\sum [n* \Delta G_(reactant)]](https://img.qammunity.org/2021/formulas/chemistry/college/3hmic8rkrj1gaaj62ijedx5bz0gwtjlswg.png)
![\Delta G_(rxn)=[(n_(CO_2)* \Delta G_(CO_2))+(n_(CaO)* \Delta G_(CaO))]-[(n_(CaCO_3)* \Delta G_(CaCO_3))]](https://img.qammunity.org/2021/formulas/chemistry/college/qrmd2aiuhao10uz88st27ztpy2ap7vev0u.png)
where,
n = number of moles
Now put all the given values in this expression, we get
![\Delta G_(rxn)=[(1* -394.4)+(1* -604.17)]-[(1* -1128.76)]](https://img.qammunity.org/2021/formulas/chemistry/college/lqywk6rqo3pvszwg7yhlbm3zbl3by5y6wi.png)

Therefore, the gibbs free energy for this reaction is, +130.19 kJ