Answer:
If the temperature was increased to 404 K, its volume would be 3.68 L.
Step-by-step explanation:
Charles' Law gives a relationship between the volume and the temperature of the gas at constant temperature. This law states that the volume of a given amount of gas held at constant pressure is directly proportional to the temperature.
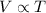
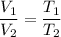
Let
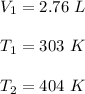
Let
 is new volume. Using above formula we get :
is new volume. Using above formula we get :
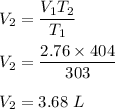
If the temperature was increased to 404 K, its volume would be 3.68 L.