Answer:
88.6%
Step-by-step explanation:
Hello,
In this case, considering the given reaction, we notice a 1:1 molar relationship between lithium hydroxide (molar mass=23.95 g/mol) and lithium chloride (molar mass=42.394 g/mol), for that reason, we are able to compute the theoretical yield of lithium chloride by stoichiometry:
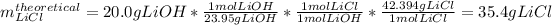
Next, by knowing the actual yield of 31.0 g, we compute the percent yield as:
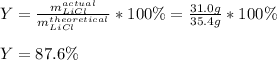
Therefore, among the given, the answer should be 88.6%
Best regards.