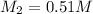Answer:
Step-by-step explanation:
Hello,
In this case, for this dilution process, we understand that the moles of the solute (potassium nitrate) remain unchanged upon the addition of diluting water. However, the resulting or final volume includes the added water as shown below:
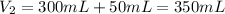
In such a way, we are able to relate the solution before and after the dilution by:
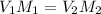
Hence, we solve for the final molarity as:
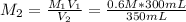
Best regards.