Answer:
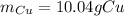
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
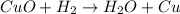
Now, since 2.844 g of water are produced, one could compute the grams of copper considering the produced water via stoichiometry and their 1:1 molar ratio:
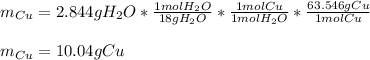
Best regards.