Answer:
Final temperature of the gas is 576
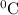 .
.
Step-by-step explanation:
As the amount of gas and pressure of the gas remains constant therefore in accordance with Charles's law:
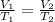
where
 and
and
 are volume of gas at
are volume of gas at
 and
and
 temperature (in kelvin scale) respectively.
temperature (in kelvin scale) respectively.
Here
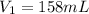 ,
,
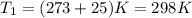 and
and
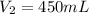
So
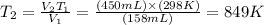
849 K = (849-273)
 = 576
= 576
So final temperature of the gas is 576
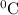 .
.