Answer:
1.76 × 10³ K
Step-by-step explanation:
Given data
- Moles of the gas sample (n): 3.63 mol
- Pressure of the gas (P): 5.36 atm
- Volume of the container in which the gas is (V): 97.77 L
- Ideal gas constant (R): 0.0821 atm.L/mol.K
For an ideal gas, we can calculate the temperature of the sample using the ideal gas equation.
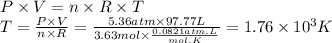
The sample is at 1.76 × 10³ K.