Answer:
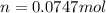
Step-by-step explanation:
Hello,
In this case, since we can consider hydrogen gas as an ideal gas, we check the volume-pressure-temperature-mole relationship by using the ideal gas equation:

Whereas we are asked to compute the moles given the temperature in Kelvins, thr pressure in atm and volume in L as shown below:
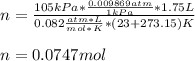
Best regards.