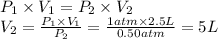Answer:
5 L
Step-by-step explanation:
Given data
- Initial pressure (P₁): 1 atm
- Initial volume (V₁): 2.5 L
- Final pressure (P₂): 0.50 atm
For a gas, there is an inverse relationship between the pressure and the volume. Mathematically, for an ideal gas that undergoes an isothermic change, this is expressed through Boyle's law.