Answer:
The age of the bones is approximately 14172 years.
Step-by-step explanation:
The age of the bones can be determinated using the following decay equation:
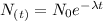 (1)
(1)
Where:
N(t): is the quantity of C-14 at time t
No: is the initial quantity of C-14
λ: is the decay rate
t: is the time
First, we need to find λ:
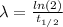
Where:
t(1/2): is the half-life of C-14 = 5730 y
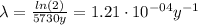
Now, we can calculate the age of the bones by solving equation (1) for t:
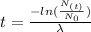
We know that the bones have lost 82% of the C-14 they originally contained, so:
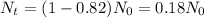
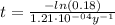
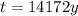
Therefore, the age of the bones is approximately 14172 years.
I hope it helps you!