Answer:
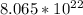 atoms of Ca.
atoms of Ca.
Step-by-step explanation:
Basic stoich question:
First the reaction is

Converting grams to moles:
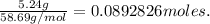
Notice the mole ratio. For every 2 Ni produced, 3 Ca was used.
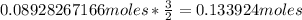 of Ca.
of Ca.
There are
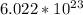 atoms in a mole.
atoms in a mole.
Thus, multiplying them together =
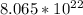 atoms
atoms