Answer:
1.25 moles
Step-by-step explanation:
First, we need to balance the equation. Essentially, this means making sure we have the same number of each atom on each side.
On the left side, we currently have:
- 1 Co atom
- 2 F aomts
On the right side, we have:
- 1 Co atom
- 3 F atoms
To balance it, add a 2 to Co on the left, 3 to F2 on the left, and 2 to CoF3 on the right:
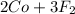 →
→
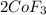
Now, we have 1.25 moles of Co, and since the ratio between Co and CoF3 is 1:1, we also have 1.25 moles of CoF3.
Thus, the answer is 1.25 moles.