Answer:
The specific heat of the metal is
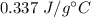 .
.
Step-by-step explanation:
We have,
Mass of sample is 22.44 g
It absorbs 180.8 J of heat.
The temperature of the sample increases from 21.1 °C to 45.0 °C
Initial temperature is 21.1 °C and final is 45.0 °C.
Heat absorbed in terms of specific heat is given by :
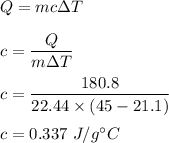
So, the specific heat of the metal is
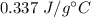 .
.