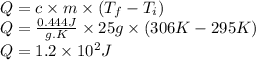Answer:
1.2 × 10² J
Step-by-step explanation:
Given data
- Initial temperature: 295 K
The amount of heat (Q) that a substance absorbs depends on its specific heat capacity (c), which is the amount of heat energy required to raise the temperature of a substance per unit of mass. It can be calculated through the following expression. (cFe = 0.444 J/g.K)