Answer: The new volume is 5 L.
Explanation: Boyle's Law describes the relationship between an ideal gas and pressure, volume and temperature, when temperature and the amount of gas are constant.
According to this law:
P₁V₁ = P₂V₂
Which states that, under those conditions, the pressure of the gas is inversely proportional to the volume.
It is also adequate to use this law when you want to determine pressure or volume at its initial or final value.
Since it is asked the final volume:
P₁V₁ = P₂V₂
V₂ =
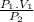
V₂ =
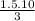
V₂ = 5
When the gas is compressed until pressure of 3 atm, its volume is 5 liters.