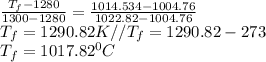Answer:
a) change in specific internal energy for the air, Δu = 675 kJ
b) Final temperature of air,
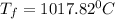
Step-by-step explanation:
The heat generated = Q
The work done = W
Time interval, Δt = 15 mins = 15 * 60
Δt = 900 s
It is stated that there is no overall change in kinetic and potential energy
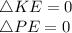
a) The change in internal energy, ΔU = Q - W
Change in specific internal energy, Δu = (Q - W)/m
Workdone is calculated by:
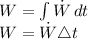
Since energy is transferred into the air, rate of energy transfer to the air is taken as negative,

W = -1 * 900
W = - 900 kJ
Energy received by heat transfer,
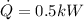
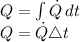
Q = 0.5 * 900
Q = 450 kJ
Mass of air, m = 2 kg
Change in specific internal energy, Δu = (Q - W)/m
Δu = (450 - (-900)/2
Δu = 675 kJ
b) By interpolation from the ideal gas property table:
At
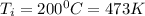
When T = 470K, u = 337.32 kJ/kg
When T = 480K, u = 344.70
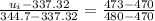
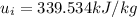
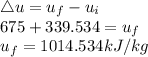
At
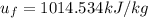
When T = 1280K, u = 1004.76 kJ/kg
When T = 1300K, u = 1022.82 kJ/kg
By interpolation,