Answer:
The volume of the piston would be 250 mL.
Step-by-step explanation:
Volume of the piston,
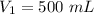
Pressure,
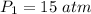
Let
 is the volume of piston if the pressure increased to 30.0 atm.
is the volume of piston if the pressure increased to 30.0 atm.
It is based on Boyle's law. It is given by:
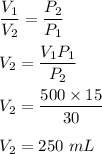
So, the volume of the piston would be 250 mL.