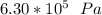Answer:
the final pressure in pascal =
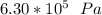
Step-by-step explanation:
Given that:
initial pressure =
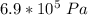
Initial Temperature = 17.5 °C = (17.5 + 273) K = 290.5 K
Volume = 2.00 L = 2.00 × 10⁻³ m³
Initial volume of the air occupied by gas V' = 95 cm³ = 95× 10⁻⁶ m
Using the ideal gas temperature;
PV = nKT
where
K = Boltzmann constant = 1.38 × 10⁻²³
From above expression;
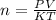
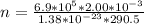
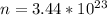
The number of moles that were removed from the tire is calculated as
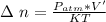
where
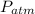 = atmospheric pressure =
= atmospheric pressure =
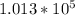
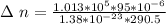
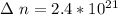
The remaining number of moles after the release of gas is
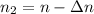
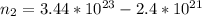
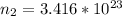
Using the expression
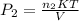 to determine the final pressure:
to determine the final pressure:
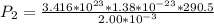
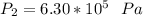
Hence, the final pressure in pascal =