Answer:
The mass of the aluminum chunk is 258 g
Step-by-step explanation:
Given;
mass of steel container = 120-g
mass of water = 150 g
initial temperature of water, = 25°C
mass of copper cube,
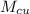 = 200 g
= 200 g
initial temperature of the copper cube,
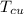 = 85°C
= 85°C
initial temperature of the aluminum chunk
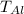 = 5.0°C
= 5.0°C
Neglecting heat loss, heat exchanged by the two metallic objects is the same since initial temperature is equal to final temperature of water.
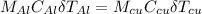
where;
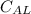 is specific heat capacity of aluminum
is specific heat capacity of aluminum
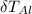 is change in temperature of aluminum
is change in temperature of aluminum
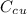 is the specific heat capacity of copper
is the specific heat capacity of copper
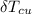 is the change in temperature of copper
is the change in temperature of copper
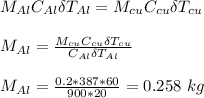
Therefore, the mass of the aluminum chunk is 258 g