Answer:
About 326 K.
Step-by-step explanation:
Recall the ideal gas law:
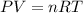
Where P is the pressure, V is the volume, n is the number of moles, R is the universal gas constant, and T is the temperature.
Because we want to solve for temperature T:
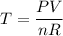
Substitute and evaluate. R has a value of 0.08206 L-atm/mol-K:

In conclusion, the temperature of the gas will be about 326 K.