Answer:
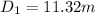
Step-by-step explanation:
We will need to use the ideal gas equation. The equation is given by:

- P is the pressure
- V is the volume
- n is the amount of molecules
- R is the ideal gas constant
As we have the same amount of molecules in the initial and final steps, therefore we can do this:
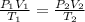 (1)
(1)
- P(1) is the atmospheric pressure (P(1) = 1 atm) and P(2) is 0.028 atm
- T(1) is 300 K and T(2) is 190 K
- V(1) is the volume of the balloon in the first step, we can consider a spherical geometry so:
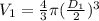 (2)
(2)
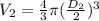 (3)
(3)
- D(2) = 32 m
So
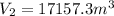
Let's solve the equation (1) for V(1)
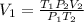
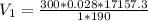
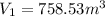
And using the equation (2) we can find D.
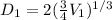
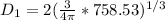
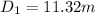
I hope it helps you!