Answer:
The correct option is;
24 moles
Step-by-step explanation:
Here, we have the reaction as follows;
Sn(s) + 2HF(g) → SnF₂ (s) + H₂ (g)
Therefore, one mole of Sn reacts with 2 moles HF to form one mole of SnF₂ and one mole of H₂
Molar mass of H₂ = 2.01588 g/mol
Therefore, the number of moles of H₂ in 48 grams of H₂ is given by the relation;
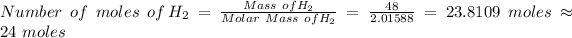
Since one mole each of SnF₂ and H₂ are produced, the number of moles of SnF₂ produced = 24 moles.
The number of moles of SnF₂ that will be produced is 24 moles.