Answer:
T = 215.33 °C
Step-by-step explanation:
The activation energy is given by the Arrhenius equation:
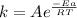
Where:
k: is the rate constant
A: is the frequency factor
Ea: is the activation energy
R: is the gas constant = 8.314 J/(K*mol)
T: is the temperature
We have for the uncatalyzed reaction:
Ea₁ = 70 kJ/mol
And for the catalyzed reaction:
Ea₂ = 42 kJ/mol
T₂ = 20 °C = 293 K
The frequency factor A is constant and the initial concentrations are the same.
Since the rate of the uncatalyzed reaction (k₁) is equal to the rate of the catalyzed reaction (k₂), we have:
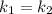
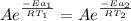 (1)
(1)
By solving equation (1) for T₁ we have:
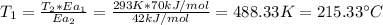
Therefore, we need to heat the solution at 215.33 °C so that the rate of the uncatalyzed reaction is equal to the rate of the catalyzed reaction.
I hope it helps you!