Answer:
A
Step-by-step explanation:
To answer this, we need to use Gay-Lussac's law, which states that:
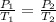 , where P is pressure and T is temperature
, where P is pressure and T is temperature
The initial pressure we're given is 4.5 atm (so P1 = 4.5) and the temperature is 45.0°C; however, we need to change Celsius to Kelvins, so add 273 to 45.0: 45.0 + 273 = 318 K (so T1 = 318).
The final pressure is what we want to find, but we do know the final temperature is 3.1°C. Converting this to Kelvins, we get: 3.1 + 273 = 276.1 K, which means T2 = 276.1.
Plug these values in:
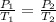
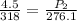
Multiply both sides by 276.1:
 ≈ 3.9 atm
≈ 3.9 atm
The answer is thus A.