Answer:
The molarity of the diluted solution is 1.786 M
Step-by-step explanation:
Dilution is a process in which you always start with a concentrated solution to which a greater volume of solvent is added. This modifies the concentration and volume of the resulting solution, but the amount of solute used to prepare the initial solution remains the same.
In summary, in a dilution the amount of solute does not vary, but the volume of the solvent varies: when more solvent is added, the concentration of the solute decreases, as the volume (and weight) of the solution increases.
The following rule is fulfilled:
Vinicial x Cinicial = Vfinal x Cfinal
where:
- Vinicial = Initial volume.
- Cinicial = Initial concentration
- Vfinal = Final volume
- Cfinal = Final concentration
In this case:
- Vinicial= 250 mL= 0.25 L (Being 1 L=1000 mL)
- Cinicial= 12.5 M
- Vfinal= 1.5 L+ 0.25 L=1.75 L
- Cfinal= ?
Replacing:
0.25 L* 12.5 M= 1.75 L* Cfinal
Solving:
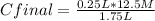
Cfinal= 1.786 M
The molarity of the diluted solution is 1.786 M