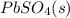Answer: The lead-containing reactant(s) consumed during recharging of a lead-acid battery is
Step-by-step explanation:
In lead acid battery, the anode is made up of lead and undergoes oxidation during discharging and cathode is made up of lead oxide and acts as cathode during discharging. The electrolyte used is dilute
 .
.
Charging:
Cathode : reduction :
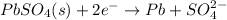
Anode: oxidation :
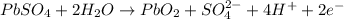
Overall reaction :
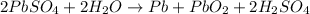
The lead-containing reactant(s) consumed during recharging of a lead-acid battery is