Answer:
a)
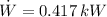 , b)
, b)
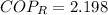 , c) Irreversible.
, c) Irreversible.
Step-by-step explanation:
a) The power input required by the refrigerator is:
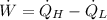
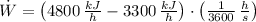
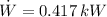
b) The Coefficient of Performance of the refrigerator is:
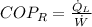
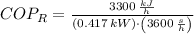
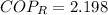
c) The maximum ideal Coefficient of Performance of the refrigeration is given by the inverse Carnot's Cycle:
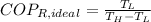
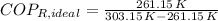
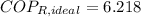
The refrigeration cycle is irreversible, as
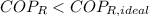 .
.