Step-by-step explanation:
We have,
The most probable speed of an oxygen molecule in the gas phase at room temperature is 450 m/s.
The formula of the most probable speed of a gas molecule is given by :
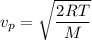
So,
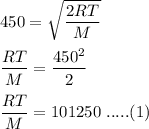
The RMS speed of the gas molecule is given by :
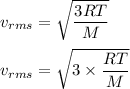
Use equation (1) in above equation :
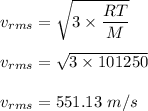
It is clear that the RMS speed of the gas molecule is greater than 450 m/s.