Answer:
77 L of water can be made.
Step-by-step explanation:
Molar mass of
 = 32 g/mol
= 32 g/mol
So, 55 g of
 =
=
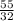 mol of
mol of
 = 1.72 mol of
= 1.72 mol of

As hydrogen is present in excess amount therefore
 is the limiting reagent.
is the limiting reagent.
According to balanced equation, 1 mol of
 produces 2 mol of
produces 2 mol of
 .
.
So, 1.72 mol of
 produce
produce
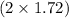 mol of
mol of
 or 3.44 mol of
or 3.44 mol of
 .
.
Let's assume
 gas behaves ideally at STP.
gas behaves ideally at STP.
Then,
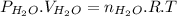 , where P, V, n, R and T represents pressure, volume, no. of moles, gas constant and temperature in kelvin scale respectively.
, where P, V, n, R and T represents pressure, volume, no. of moles, gas constant and temperature in kelvin scale respectively.
At STP, pressure is 1 atm and T is 273 K.
Here,
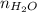 = 3.44 mol and R = 0.0821 L.atm/(mol.K)
= 3.44 mol and R = 0.0821 L.atm/(mol.K)
So,
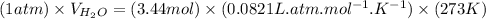

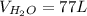
Option (b) is correct.