Answer:
Products are stearate anion and water.
Step-by-step explanation:
Stearic acid is a 18-carbon chain molecule containing -COOH group. IUPAC name of stearic acid is octadecanoic acid.
Molecular formula of stearic acid is
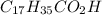 .
.
When
 is added into stearic acid,
is added into stearic acid,
 removes a proton (
removes a proton (
 ) from acidic -COOH group and forms stearate anion and water as products.
) from acidic -COOH group and forms stearate anion and water as products.
The balanced acid-base reaction is given as:
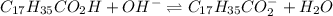
Structure of products are given below.