Answer:
No.
Step-by-step explanation:
The Coefficient of Performance of the reversible heat pump is determined by the Carnot's cycle:
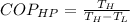
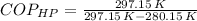
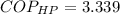
The power required to make the heat pump working is:
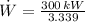
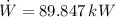
The heat absorbed from the exterior air is:
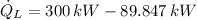
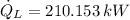
According to the Second Law of Thermodynamics, the entropy generation rate in a reversible cycle must be zero. The formula for the heat pump is:
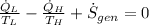
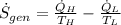
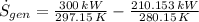
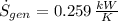
Which contradicts the reversibility criterion according to the Second Law of Thermodynamics.