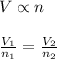Answer :
(3)
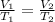
(4)
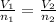
(5)

(7)
Explanation :
Boyle's Law : It is defined as the pressure of the gas is inversely proportional to the volume of the gas at constant temperature and number of moles.
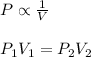
Charles' Law : It is defined as the volume of the gas is directly proportional to the temperature of the gas at constant pressure and number of moles.
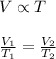
Gay-Lussac's Law : It is defined as the pressure of the gas is directly proportional to the temperature of the gas at constant volume and number of moles.
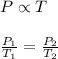
Avogadro's Law : It is defined as the volume of the gas is directly proportional to the number of moles of the gas at constant pressure and temperature.