Question:
a. a direct linear relationship
b. an inverse linear relationship
c. a direct nonlinear relationship
d. an inverse nonlinear relationship
Answer:
The correct option is;
d. An inverse nonlinear relationship
Step-by-step explanation:
From the universal gas equation, we have;
P·V = n·R·T
Where we have the temperature, T and the number of moles, n constant, therefore, we have
P×V = Constant, because, R, the universal gas constant is also constant, hence;
P×V = C
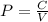
Since P varies with V then the graphical relationship will be an inverse nonlinear as we have
V P C
1 5 5
2 2.5 5
3 1.67 5
4 1.25 5
5 1 5
6 0.83 5
7 0.7 5
8 0.63 5
9 0.56 5
10 0.5 5
Where:
V = Volume
P = Pressure
C = Constant = 5
P = C/V
The graph is attached.