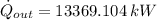Answer:
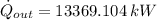
Step-by-step explanation:
The turbine is modelled after the First Law of Thermodynamics:
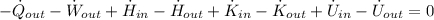
The rate of heat transfer between the turbine and its surroundings is:
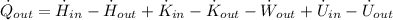
The specific enthalpies at inlet and outlet are, respectively:
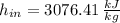
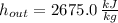
The required output is:
![\dot Q_(out) = \left(8\,(kg)/(s) \right)\cdot \left\{3076.41\,(kJ)/(kg)-2675.0\,(kJ)/(kg)+(1)/(2)\cdot \left[\left(65\,(m)/(s) \right)^(2)-\left(42\,(m)/(s) \right)^(2)\right] + \left(9.807\,(m)/(s^(2)) \right)\cdot (4\,m) \right\} - 8000\,kW](https://img.qammunity.org/2021/formulas/engineering/college/i75ag8y6h0allcj23x54663x3jmjgchubj.png)