Answer:
The volume that the gas will occupy when the temperature is increased to 400.0 K and the pressure is increased to 200. kPa is 4000. L
Step-by-step explanation:
Here we are required to utilize the combined gas equation as follows;
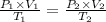
Where:
P₁ = Initial pressure of the gas = 100.0 kPa
V₁ = Initial volume of the gas = 2000. L
T₁ = Initial temperature of the gas = 100.0 K
P₂ = Final pressure of the gas = 200.0 kPa
V₂ = Final volume of the gas = Required
T₂ = Final temperature of the gas = 400.0 K
Making V₂ the formula subject of the combined gas equation, we have;
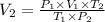
Therefore, by plugging the values, we have;
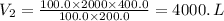
The volume that the gas will occupy when the temperature is increased to 400.0 K and the pressure is increased to 200. kPa = 4000. L.