Answer:
1.25 Kcal
Step-by-step explanation:
The relationship between heat and temperature change is given by the equation:
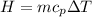
where H = heat energy (Joules, J) , m = mass of a substance (kg) , c = specific heat (units J/kg∙K) and ΔT is the change in temperature
Given that:
mass of water (m) = density of water × volume = 1g/ml × 50ml = 50 g, ΔT = 25° C and
 of water= 4.18J/g/°C
of water= 4.18J/g/°C
Therefore H = 50g × 4.18J/g/°C × 25°C = 5225 J
The heat transferred to the Jelly bean = 5225 J = 1.25 Kcal