Answer:
The molarity of a 50.0 mL solution that contains 0.0350 mol of sodium sulfate 0.7

Step-by-step explanation:
Molarity is a concentration measure that indicates the amount of moles of solute that appear dissolved in each liter of the mixture.
So, the Molarity of a solution is determined by the expression:
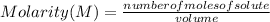
Molarity is expressed in units (
 ).
).
In this case:
- Volume=50 mL=0.05 L (1 L= 1,000 mL)
Replacing:
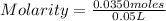
Molarity= 0.7

The molarity of a 50.0 mL solution that contains 0.0350 mol of sodium sulfate 0.7
