Answer: The given sample of water is not safe for drinking.
Step-by-step explanation:
We are given:
Concentration of fluorine in water recommended = 4.00 ppm
ppm is the amount of solute (in milligrams) present in kilogram of a solvent. It is also known as parts-per million.
To calculate the ppm of fluorine in water, we use the equation:
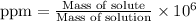
Both the masses are in grams.
We are given:
Mass of fluorine =
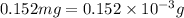 (Conversion factor: 1 g = 1000 mg)
(Conversion factor: 1 g = 1000 mg)
Mass of water = 5.00 g
Putting values in above equation, we get:
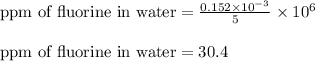
As, the calculated concentration is greater than the recommended concentration. So, the given sample of water is not safe for drinking.
Hence, the given sample of water is not safe for drinking.