Answer: The half-cell reaction occurring at cathode is
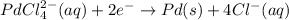
Step-by-step explanation:
Oxidation reaction occurs at anode and reduction reaction occurs at cathode.
Reduction reaction is defined as the reaction in which a chemical specie accepts electrons. The oxidation state of the chemical specie reduces.
The given balanced chemical equation is:
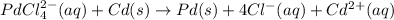
The half cell reaction occurring at cathode follows:
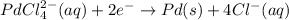
Hence, the half-cell reaction occurring at cathode is given above.