Answer:
the concentration of the Zn²⁺ (aq) ion at the cathode is 0.255 M
Step-by-step explanation:
The voltage generated by the zinc electric cell that is discribed by the following relation;
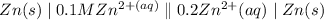
The Nernst equation is given as follows;
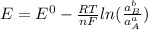
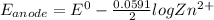
![E_(anode)-E_(cathode) =-(0.0591)/(2) log([Z^(2+)])/([x])](https://img.qammunity.org/2021/formulas/chemistry/high-school/c6ryvt6k2lkwn1jqwlp335prtiek4m3b3i.png)
![0.012 =-(0.0591)/(2) log(0.1)/([x])](https://img.qammunity.org/2021/formulas/chemistry/high-school/tdmc47buur5pffqesus7fm8nfob0iekxx7.png)
x = 0.255 M.
Therefore the concentration of the Zn²⁺ (aq) ion at the cathode = 0.255 M.