Answer:
The molarity is 0.189

Step-by-step explanation:
Molarity is a measure of concentration of a solution that indicates the number of moles of solute that are dissolved in a given volume. In other words, the molarity indicates the amount of moles of solute that appear dissolved in each liter of the mixture.
Molarity is determined by the expression:
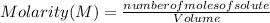
Molarity is expressed in units (
 ).
).
So, you need to know the amount of moles of ZnSO₄. Then you must know the molar mass. If:
- Zn: 65 g/mol
- S: 32 g/mol
- O: 16 g/mol
the molar mass of ZnSO₄ is:
ZnSO₄= 65 g/mol + 32 g/mol + 4* 16 g/mol= 161 g/mol
Then you can apply a rule of three as follows: if 161 g of ZnSO₄ is contained in 1 mole, 13.7 g in how many moles are they?
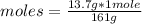
moles=0.085
Then you know:
- number of moles of solute= 0.085 moles
- Volume= 450 mL (Being 1 L=1,000 mL)
Replacing in the definition of molarity:
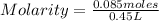
Molarity=0.189

The molarity is 0.189
