Answer:
The answer is 20.6 grams.
Step-by-step explanation:
Molality describes the concentration of a solution. It can be defined as the number of moles of a solute dissolved in 1 kilogram of solvent. Then it is equal to the moles of solute (the substance that dissolves) divided by the kilograms of solvent (the substance used to dissolve):
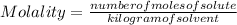
The molality is expressed in units (
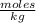 ).
).
So, you can apply the following rule of three with the solution being 0.5 molal: if in 1 kg of solution there are 0.5 moles of solute, in 0.4 kg (400 g, being 1kg = 1000g) how many moles of solute are there?
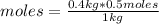
moles=0.2 moles
Now, you know:
- Na: 23 g/mole
- Br: 80 g/mole
Then, The molar mass of sodium bromide NaBr is
NaBr= 23 g/mole + 80 g/mole= 103 g/mole
Now a new rule of three applies, if in 1 mole of sodium bromide there are 103 grams, in 0.2 mole how much mass is there?
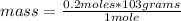
mass= 20.6 grams
The answer is 20.6 grams.