Answer:
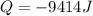
Step-by-step explanation:
Hello,
In this case, the released heat by the water in this heating process is defined in terms of its mass, heat capacity and temperature difference as shown below:
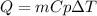
Thus, with the given data indicating that water was at 55 °C before entering the body, we obtain:
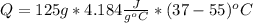
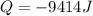
Such negative result means that the water releases that amount of heat to the body.
Best regards.