Answer:
Cations (positively-charged): With a superscript + (e.g.
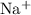 )
)
Anions (negative-charged): With a superscript - (e.g.
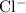 )
)
Step-by-step explanation:
An ion is an atom that carries some sort of negative or positive charge due to losing or gaining electrons in bonding with another atom. A cation, a positively-charged ion, loses an electron in the bonding process, and is written with a small plus sign superscript, while an anion, a negatively-charged ion, is written with a small minus sign. For example, a sodium cation in a sodium chloride molecule would be written like
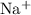 , and the corresponding chloride anion would be written like
, and the corresponding chloride anion would be written like
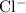 )
)