Answer: The volume of 0.684 mol of carbon dioxide at s.t.p. is 15.3 L
Step-by-step explanation:
According to ideal gas equation:
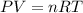
P = pressure of gas = 1 atm (at STP)
V = Volume of gas = ?
n = number of moles = 0.684
R = gas constant =
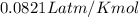
T =temperature =
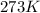 (at STP)
(at STP)
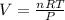
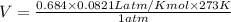
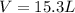
Thus the volume of 0.684 mol of carbon dioxide at s.t.p. is 15.3 L